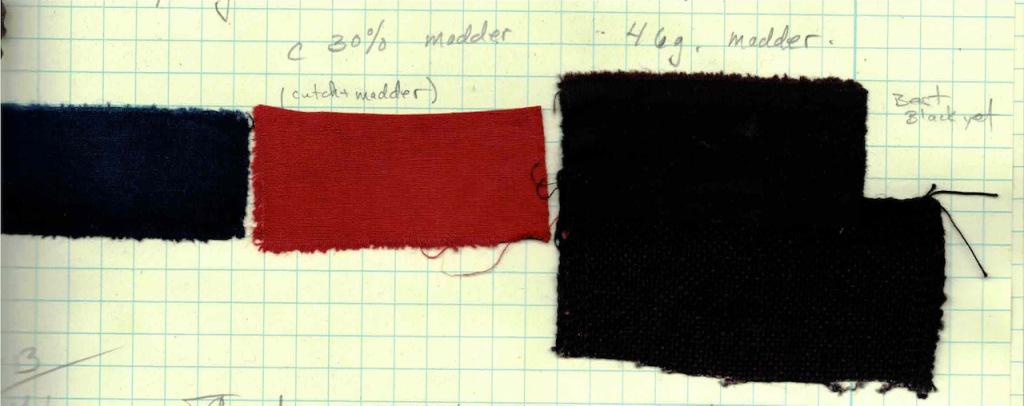
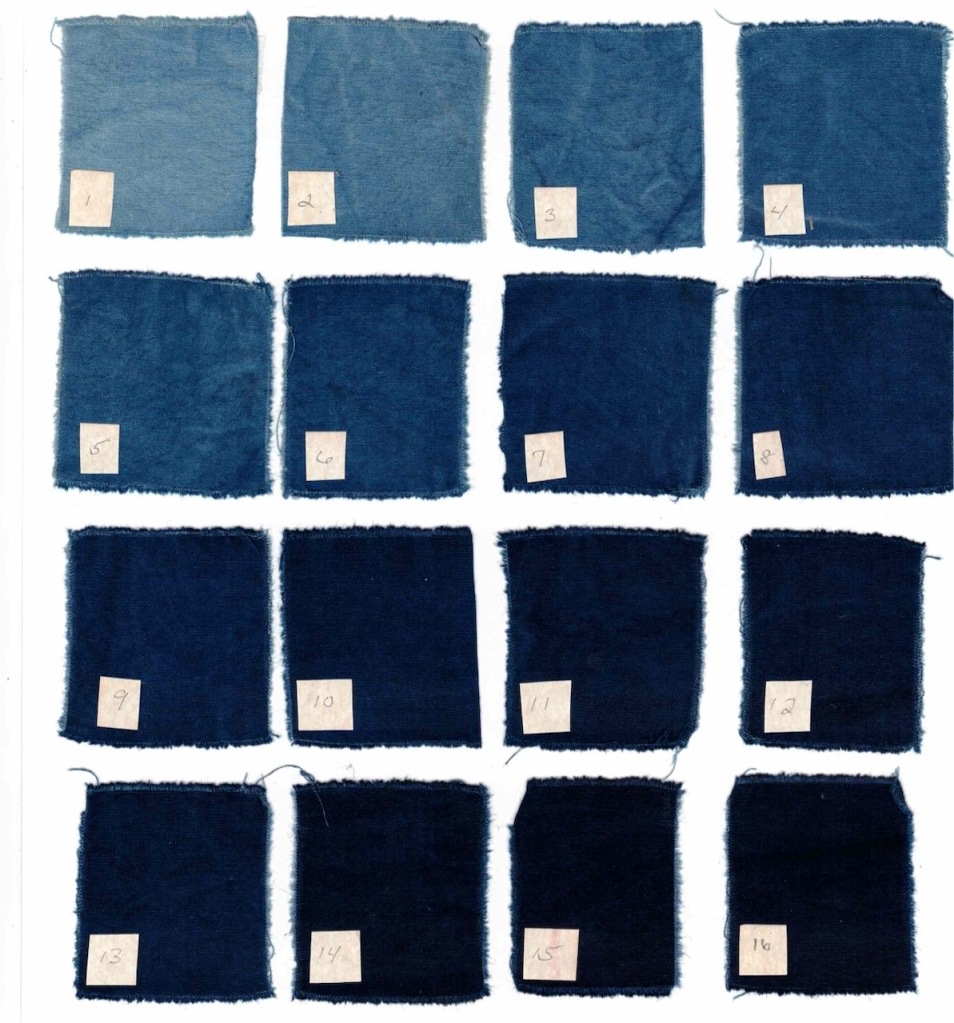
I am always trying to learn more about the natural dye process. Mostly, this is to become a better dyer, but there is also an intellectual curiosity that keeps me asking questions, testing, and observing. Joy Boutrup, my co-author of The Art and Science of Natural Dyes, is a textile chemist and engineer. She has generously explained so much to me over the years, giving me a glimpse of the logic behind all of our dyeing processes, but never overwhelming me with the technicality of the chemistry.
Last summer, Joy and I were teaching at Penland School of Crafts at the same time as Tim McLaughlin and Charllotte Kwon of Maiwa. It was there I learned of the chemistry class for dyers that they had been developing. In a desire to learn more and expand my ability to understand process, I enrolled in the class, Natural Dyes: Alchemy Chemistry Craft when it was offered for the first time. The class is presented in 18 separate modules, each a seriously “deep dive” into electrons, molecules, acids, bases, and the nature of plants that we use in our processes. Each lesson is presented in video format by Tim and Charllotte with printable notes that link directly to the video script.
To be honest, much of it was over my head, as I do NOT have a chemical background.
The class is not a practical “how-to” of dyeing, but instead, a serious look into the principles behind the process. As the lessons progressed, I found myself making connections and the processes that I know well were explained even further. There is a reason for each of the steps we use in the dye process, whether we see them, understand them, or not.
Several of my colleagues also took the class. Early this year, when the class was offered for a second time, several other colleagues enrolled. So, a small group of 7 serious and curious dyers decided that we would go through the class together. This would give us an opportunity to discuss, compare notes, and work through the class together. An enrolled student has three years to access the videos but the printable notes are yours forever, making them a valuable studio reference.
Our “Study Group” members are located all across the US. We opted to do our watching/reading separately and come together once a week via zoom to discuss a single module.
We tried this for a few weeks, but then decided that we needed a deeper focus to smaller parts of each module. Each of us now takes on the responsibility to further explore, research, and understanding one (or two) aspects of a module. This allows for more in-depth research, more focused questions, and possible dye/process testing and observing. When we complete this series of modules, I do believe that we could start all over again, and still learn more about the dye/chemical process.
Understanding and practicing natural dye is a never ending process. Learning WHY we do things is key to being our best as dyers.
During the first chemistry class in the fall of 2023, we received the shocking and sad news that Tim McLaughlin had passed away very suddenly. No one in the Maiwa family or in our larger community of dyers was prepared for this. It made continuing with the class difficult at that time, but now I feel that we are honoring Tim by continuing to learn from what he has created. We miss him dearly.
I am awe of the work that Tim and Charllotte put into this class – in order to make all of us better dyers.
Maiwa is offering the dye chemistry class once again this spring…. Serious dyers should consider taking this class and I would highly recommend working with a group of colleagues in the process.
Link to Maiwa Teachable: Natural Dyes: Alchemy Chemistry Craft


Tim and Charllotte taught an ink making class at Penland School of Crafts in the summer 2023. At the same time, Joy and I were teaching a weaving and natural dye class together. We had so much to discuss after long days in the studios! This is where I first learned of Maiwa’s upcoming science class for dyers.