© Catharine Ellis, as posted to the blog: Natural Dye: Experiments and Results
The chemical formula of indigo is C16H10N2O2
Why is reduction called “reduction”?
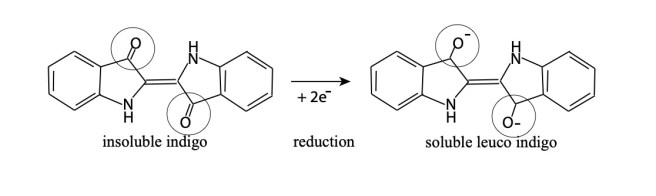
In the early days of chemistry, oxidation was defined as a gaining of oxygen atoms, and reduction was a loss of oxygen atoms. Indigo was said to be reduced because it lost an oxygen atom.
This is one of the grand “mysteries” of dyeing and chemistry!
Leucoindigo is visible as the characteristic yellowish color below the surface of some vats. The leuco color of fermentation vat is more green than yellow. Once a textile is immersed in the vat it come out of that vat with the leuco color (yellow or green). The textile will turn blue through exposure to oxygen.
What is Redox?
Redox is a chemical reaction that takes place between an oxidizing substance and a reducing substance. The oxidizing substance loses electrons in the reaction, and the reducing substance gains electrons. These two things happen simultaneously, and one does not happen without the other
All indigo vats require a high alkalinity (high pH) for proper functioning. The plants, carbohydrates, sugars, or minerals used for the indigo vat are reductive, which means that they oxidize and give off electrons. In the alkaline environment of the vat, reduction is even stronger.
The indigo molecule is forced to receive the two negatively charged electrons, which is a reduction; this influences the oxygen bonds of the indigo pigment, making the indigo molecule attractive to the positively charged portion of the water molecule. In this way the indigo becomes soluble (leucoindigo). Once it is soluble, the dye can penetrate the textile. After dyeing, oxidation (exposure to air) will once again make the indigo insoluble in the textile.





Catherine, I think you have a typo
Takes me back to organic chemistry class….
It’s so interesting–the different shades from the fermented vs. the fructose vat. Thanks for all this info. I have been growing Japanese indigo and learning the pigment extraction process. This year I plan on tackling the vat process. I tried last year, without success, so… Wish me luck!